Graphene-like molecules (GLMs) generally feature extraordinary electronic, thermal and mechanical properties that make them promising candidates for practical applications in electronics, sensing, catalysis, energy storage and conversion. Numerous experimental and theoretical results have indicated that the properties of the GLMs primarily rely on their geometric structures. For instance, GLMs with a zigzag edge support a localized edge state, whereas armchair-edged GLMs do not. Although progress has been made for the preparation of armchair-edged GLMs, a general synthetic strategy to zigzag type remains elusive.
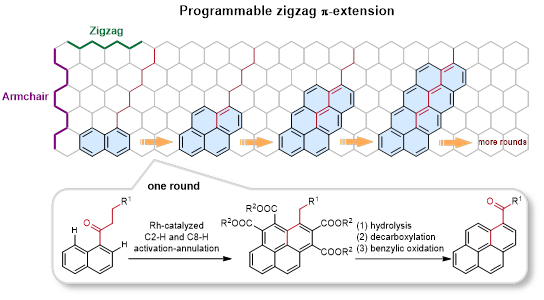
In recent years, aryl alkyl ketones have attracted interest in the C-H activation field because of their weak coordinating capacity and keto-enol tautomerism. Prof. You’s group have developed C-H activation/annulation reactions of aryl alkyl ketones with alkynes to access structurally diverse pure carbon and oxonium doped polycyclic aromatic hydrocarbons. (Angew. Chem. Int. Ed., 2021, 60, 12371;Angew. Chem. Int. Ed., 2019, 58, 302;Nat. Commun., 2019, 10, 5664;Angew. Chem. Int. Ed. 2017, 56, 13094;Angew. Chem. Int. Ed., 2017, 56, 4286;Nat. Commun., 2014, 5, 5030). Inspired by these results, You’s group reported a rapid and modular strategy to extend the π-conjugation in a zigzag fashion through a rhodium-catalyzed sequential C2-H and C8-H activation/annulation of naphthalene ketones with acetylenedicarboxylates as the C2 insertion unit. This programmable zigzag π-extension can be compared to the stacking of toy blocks, as the naphthalene fragments are added step by step along an alkyl chain. Control experiments and DFT calculations indicated that different from the reported C–H activation-annulation of aryl ketones with alkynes to form a fve-membered indenol, fulvene or six-membered pyran oxonium, this [4 + 2] and [4 + 2] annulation sequence selectively undergoes C(sp3)–H cyclization to extend the naphthalene fragment. This synthetic toolbox has been demonstrated to be compatible with a broad range of functional groups and π-conjugated skeletons. Meanwhile, these zigzag π-extension products were easily transformed by hydrolysis and condensation to deliver n-type organic semiconductors for OTFT applications and TADF materials for OLED applications. The test results demonstrate that the synthetic strategy has great potential in the preparation of high-performance OTFT and OLED materials.
This work is published on Nature Synthesis, titled as “Programmable zigzag π-extension toward graphene-like molecules by the stacking of naphthalene blocks”. Sichuan University is the top institute in the address list. Prof. Jingsong You (Sichuan University) and Prof. Yu Lan (Chongqing University) are the corresponding authors, Dr. Jiangliang Yin and Jian Li contribute equally to this work as co-first authors. This work is financially supported by the National NSF of China and the Office of China Postdoctoral Council, the International Postdoctoral Exchange Fellowship Program.
Link: https://doi.org/10.1038/s44160-023-00306-6
