Axially chiral heteroaryl molecules are of central importance in synthetic chemistry and pharmaceutical industry, not only owing to their wide applications as chiral ligands, organocatalysts and materials, but also for their multitudinous bioactive properties. Among them, the six-membered axial heteroaryl backbones particularly connected by C–C axes are the most common and well established. However, the construction of five-membered heteroatropisomeric structures with C-C axis is correspondingly rare, probably due to the perception of comparatively lower rotation barriers to that of the six-membered biaryls.
Recently, the Wang group developed the first organocatalytic asymmetric approach towards the construction of axially chiral arylpyrazole skeletons with phosphorus unit via a novel cascade reaction proceeding through Huisgen-type cycloaddition/aromatization with central-to-axial chirality conversion by dipeptide-phosphonium salt catalysis. This reaction provides a general and modular platform to access a great diversity of axially chiral arylpyrazole-based phosphorus compounds in high chemical yields with excellent enantioselectivities under very low catalyst loading (1 mol%). The practicality and utility of this method were demonstrated by the gram-scale synthetic reactions and facile elaborations, particularly towards preparing novel axially chiral arylpyrazole phosphines, which were successfully employed as chiral ligands in some selected asymmetric reactions, including Pd-catalyzed asymmetric allylic alkylation/amination and Rh-catalyzed asymmetric 1, 4-addition. Moreover, mechanistic investigations elucidated the origins of asymmetric induction.
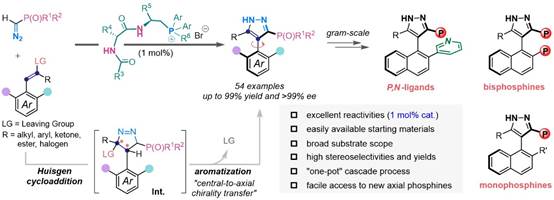
This work was recently published on Angewandte Chemie International Edition with the title "Towards Axially Chiral Pyrazole-Based Phosphorus Scaffolds by Dipeptide-Phosphonium Salt Catalysis"(URL:https://onlinelibrary.wiley.com/doi/10.1002/anie.202215720). Sichuan University is the first unit, Professor Tianli Wang of the College of Chemistry is the first corresponding author, and the doctoral student Jiahong Wu, visiting scholar Dr. Jian-Ping Tan and the graduate student Jiayan Zheng are co-first authors of the paper.We acknowledge financial supports from National Natural Science Foundation of China, National Key R&D Program of China, Fundamental Research Funds from Sichuan University, Beijing National Laboratory for Molecular Sciences, National Natural Science Foundation of Hunan, and Fundamental Research Funds for the Central Universities. We also acknowledge the College of Chemistry and the Analytical & Testing Center of Sichuan University, and particularly we thank Dr. Jing Li and Dr. Dongyan Deng from the College of Chemistry Sichuan University for HRMS and NMR testing, respectively.
