The Nobel Prize in chemistry in 2022 was awarded to Carolyn R. Bertozzi (An American chemist), Morten Meldal (A Danish chemist) and K. Barry Sharpless (An American chemist) “for the development of click chemistry and bioorthogonal chemistry”. Its fundamental value in constructing engineered biological materials and for exploring mystery in chemical biology cannot be overestimated. “Click” chemistry is a kind of convenient chemical reactions that can efficiently and rapidly build artificial molecular entities, capable of achieving selective covalent ligation in a biocompatible environment, while “photoclick” chemistry combines the concepts of “click” chemistry and “photo-controlled” reactions in a single system. Therefore, it inherits the high spatiotemporal resolution of a photo-controlled reaction and can be widely used for the lithographic surface functionalization of bio-based interfaces, for in situ 3D photo-printing of biomaterials as well as for labeling and tracking of biomolecules in native cellular environments. In contrast to click chemistry, the highly reactive intermediates generated after photo-stimulation not only be able to covalently couple with immobilized target reporter molecules, but are also prone to many side reactions, resulting in “off-target” labeling with reduction in the accuracy of the spatiotemporal resolution.
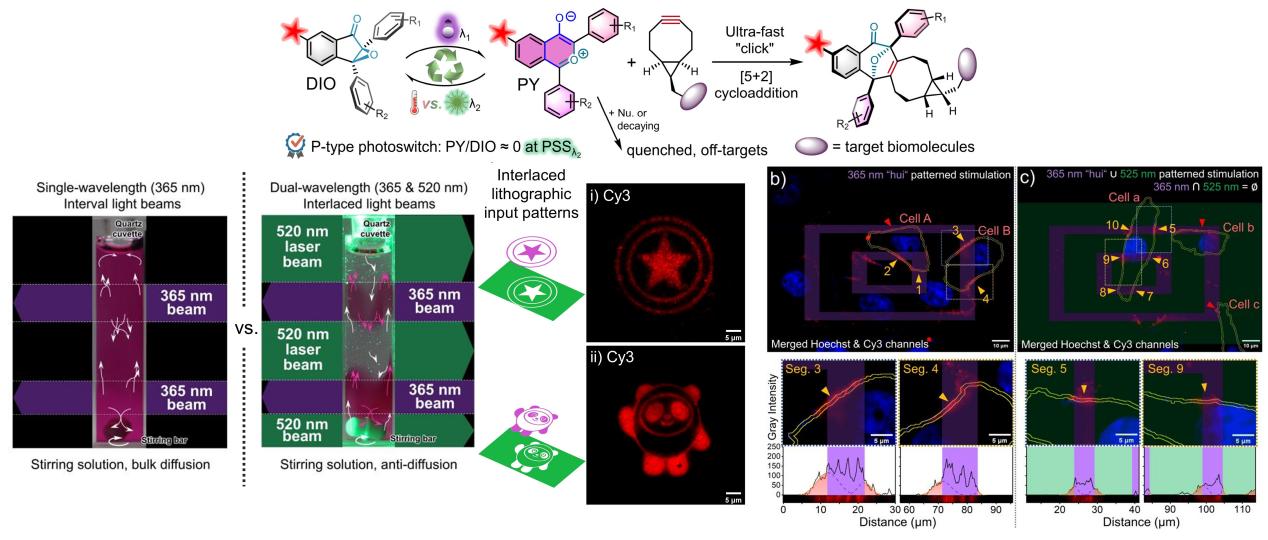
Recently, Prof. Zhipeng Yu’s group has reported an intriguing research, in which the “Diaryl Indenone Epoxide (DIO)–Oxidopyrylium Ylide (PY)” molecular photoswitch was exploited as a novel type of photoclick reaction, and an effective suppression of side-reactions with extremely high-resolution labeling on glass or cell membranes was achieved via a spatiotemporal dual-wavelength lithographic technology. As a P-type molecular photoswitch, the distribution and ration of the DIO↔PY can be strictly managed by either 365 or 520 nm photo-stimulation, which enables regeneration of the reactive PY dipole in designated space at specific time. The photo-generated PY dipole can undergo ultra-fast [5+2] cycloaddition reactions with ring-strained alkenes/ynes, representing by endo-BCN with the second-order reaction rate up to 1.9 × 105 M-1s-1. Accordingly, a brand-new fluorophore-DIO conjugate probe was synthesized for fluorescence imaging purposes. Therefore, submicron resolution with high-contrast fluorescence pattered decoration on a mono-molecule layer (BCN monolayer) coated on glass surface could be obtained in aqueous medium by taking advantages of this novel photoclick reaction, utilizing a cycled lithographic patterning with a 365 nm LED light in combination with a 525 nm complementary lithographic depletion. The lithographic cycles were programmed through an embedded Digital Micromirror Device (DMD) on an inverted microscope for convenient operation. A modified chimeric mAb, cetuximab, with a BCN reporter was established for immuno-recognition by epidermal growth factor receptor (EGFR+) receptors on living A549 cells for cellular incorporation of the bioorthogonal reporter. Therefore, spatiotemporal-resolved fluorescence labeling toward the receptors on living cell membrane was demonstrated by implement a 365 nm stimulation with a pattern of Chinese "hui" character. Noticeably, the authors also developed an algorithm to quantify the diffusion distance for the fluorescence labeling outside the illuminated region on the cell-membrane segment, clarifying the resolution of the photoclick reaction. When appending a 525 nm stimulation pattern in complementary to the "hui" character in a stimulation sequence, the diffusion distance on living cell-membrane is much shorter than when only 365 nm stimulation is available. Therefore, it is of breakthrough that the photoswitchable dipole can be modulated by a cycled dual-wavelength lithographic technique to alleviate the “off-site” labeling by the diffusion of the reactive intermediates, thereby exhibiting significant improvement on the spatial resolution of the imprinted patterns on cell membranes or glass surface. This research presents a conceptual innovation for photoclick chemistry with great application prospects.
This work was recently published on Angewandte Chemie International Edition with the title "Photoswitchable Oxidopyrylium Ylide for Photoclick Reaction with High Spatiotemporal Precision: A Dynamic Switching Strategy to Compensate for Molecular Diffusion" (URL:https://onlinelibrary.wiley.com/doi/10.1002/anie.202300034). Sichuan University (SCU) is the only research institute, Professor Zhipeng Yu from the College of Chemistry at SCU is the only corresponding author, and the doctoral student, Xinyu Xie, is the first author for accomplishing the research. The authors acknowledge financial supports from National Natural Science Foundation of China, the Fundamental Research Funds for the Central Universities and the Fundamental Research Funds from Sichuan University. The authors also express gratitude to Dr. Pengchi Deng (Analytical & Testing Center, SCU) for help on NMR spectra collection. The authors appreciate the Xiaoming Feng laboratory (SCU) for access to equipment. The authors are grateful to Yanhong Liu (the Comprehensive Training Platform of Specialized Laboratory, College of Chemistry) for her assistance with the confocal image collection.
